User Tools
Sidebar
Table of Contents
Brief introduction to NanoSIMS
NanoSIMS is an ion imaging technique that allows the analysis of elemental and isotopic composition of a solid sample at a sub-micrometer spatial scale (down to 50 nm). Although originally “NanoSIMS” was the name of an instrument that makes such an analysis possible (NanoSIMS 50 or 50L, produced by Cameca), it is now commonly used as a synonym for the technique itself. An informative overview of the NanoSIMS technique, together with many examples of applications ranging from cell biology to microbial ecophysiology to cosmochemistry, can be found on the Cameca website or in a number of review articles, e.g., Lechene et al. (2006), Wagner (2009), Hoppe et al. (2013).
Basic principle
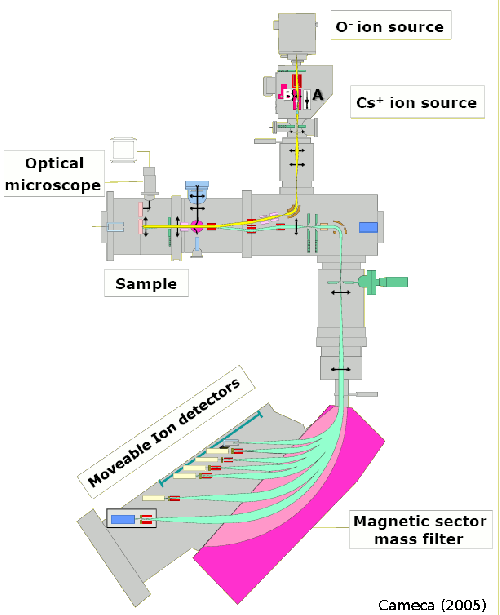
NanoSIMS analysis uses a finely focused beam of ions (ion probe) to erode the sample surface. The secondary ions (atomic and molecular) that are produced in this process are then analyzed by a mass spectrometer.
Although the basic principle is rather simple, the technical realization for achieving high sensitivity, mass resolution and spatial resolution requires some very sophisticated technology. This technology is put together in an instrument called NanoSIMS 50 or 50L, produced by Cameca. Aspects of the instrument that are important from the perspective of the sample preparation and measurements performed at the Utrecht University are briefly discussed below.
Things to remember about the measurement
Versatile but destructive
Since any material will be eroded when hit by high-energy ions, NanoSIMS can be used to analyse practically any kind of solid material. On the other hand, because of this erosion, the technique is destructive. For example, when analysing mineral particles of about 100 nm in size, the particles will be eroded away in a few seconds. When analysing microbial cells, which are typically 1-2 microns in size, they will be eroded away on the scale of minutes.
High spatial resolution
Spatial resolution of ~50 nm is achieved by using a focused ion beam that hits the sample surface perpendicularly to its surface. By rastering such a beam over the sample surface, high-spatial resolution images can be obtained. Clearly, the sample position must be highly stable to achieve images with accurate spatial information. Here, temperature control of the laboratory is essential to minimize distortions of the images, which is important especially when taking multiple planes of the same field of view.
High mass resolution
The mass spectrometer used for the analysis has a very high mass resolution (M/dM>5000). This allows discrimination between ions of very close masses. For example, when analyzing organic material enriched in 15N, one must be able to distinguish the molecular ion 12C15N- (mass 27.000) from the molecular ions 13C14N- (mass 27.006) and 12C14N1H- (mass 27.011), as these are all likely eroded simultaneously from the sample. To be able to do this, mass resolution (M/dM) of better than 27/0.006=4500 is required.
Simultaneous measurement of up to 7 masses
NanoSIMS 50/50L uses a magnet to separate the masses. According to the law of electromagnetism, namely the Lorenz magnetic force, the trajectory of a charged particle traveling in a magnetic field is curved, with the curvature proportional to the ratio between the particle's mass and charge (m/q). After passing through the magnet, particles with different m/q ratios will emerge at different locations, where they can be detected by sensitive detectors. NanoSIMS 50L has 7 detectors, which makes it possible to study co-localization of 7 masses in the sample.
Wide range of detected masses, but ...
In principle, atomic or molecular ions with masses ranging from hydrogen to uranium and beyond can be detected by NanoSIMS. However, there are some restrictions with respect to the simultaneous detection of multiple masses.
- Because of the finite size of the magnet, the minimum and maximum masses that can be analyzed simultaneously cannot differ by more than a factor of 22. For example, masses ranging from 1 amu to 22 amu can be detected simultaneously, but if you want to detect sulphur (mass 32 amu), you cannot simultaneously detect hydrogen (mass 1 amu).
- The detected elements need to be ionized. Because some elements are preferably ionized as positive ions while others as negative ions, it is possible to simultaneously detect only a combination of elements that ionize either as positive or negative ions.
- Some elements are very difficult to ionize, and are therefore preferably detected as molecular ions (e.g. N is detected as CN-). This brings along problems with interferences. For example, when analyzing N as 12C14N- (mass 26.0031) from an organic material, molecular ions 13C13C- (mass 26.0067) and 12C13C1H- (mass 26.0112) will also be eroded simultaneously from the sample. Therefore, if the sample is substantially enriched in 13C, it can cause difficulties in accurate detection of its nitrogen content or isotopic composition.
- Due to the finite physical size of the detectors and the need to place them next to each other when detecting similar masses, there is a limit with respect to the separation of masses that can be detected simultaneously. An approximate formula for calculating the difference in masses that can be detected simultaneously is dM = sqrt(Mmax x M) x 0.017, where Mmax is the maximum detected mass and M is the base mass for which one wants to simultaneously detected also the mass M+dM. For example, if the maximal detected mass is set to Mmax=127 amu, and one wants to detect mass 12 amu (12C), one cannot detect masses that are closer to 12 amu than dM=0.66 amu. Since the distance between 12C and 13C is 1 amu, their simultaneous detection together with mass 127 is possible. (Note that dM in this paragraph should not be confused with the mass resolution mentioned above.)
Probing ions: Cs+ vs. O-
Experiments showed that the efficiency of emission of ionized particles differs if the surface bombardment is done with different primary ions. The best efficiency is achieved by using Cs+ for detecting negative ions and O- for detecting positive ions. Although both primary ion sources are available with the NanoSIMS 50/50L instrument, only one can be used at a time. The fact that switching to a different source is a somewhat lengthy procedure imposes some constrains on the experimental design and the subsequent NanoSIMS analysis. Since 2020 the facility has been upgraded with a new generation oxygen source: the Hyperion H201 ion source. This upgrade makes measurements with a higher resolution and a higher brightness possible for samples that need to be measured with oxygen ions.
Complex ion optics
To achieve maximum sensitivity of the instrument, many components of its ion optics need to be fine-tuned. Since a number of these optimization steps need to be done frequently, even when moving from one field of view on the sample to another, the total analysis of a batch of samples (replicates+treatments) from a given experiment may take some time (several days of continuous measurement time). Clearly, the experimental design should consider this very carefully.
Things to remember when preparing samples
Sample charging
Since the detection by NanoSIMS is achieved through bombardment with ions, net charge would be deposited onto the sample during the measurement if the charge was not removed. Since most of the analyzed samples will not be sufficiently conductive, samples should be coated with a thin conductive layer (e.g. Au, C, Au-Pd), which will facilitate removal of the excess charge and thus prevent problems associated with sample charging. For samples that are put on a support substrate (e.g. cells or tissues on a filter or a glass slide), the conductive layer deposition can be done before or after the sample deposition. A gold-coating instrument is available at Utrecht University.
Flat sample surface
Experiments showed that the detected signal is affected by minute changes in topography of the sample surface. To minimize these artifacts, samples should be prepared as flat as possible. For geological samples, polishing with a 30-50 nm powder is advised. Polishing instruments are available at Utrecht University.
Thin sample sections
As mentioned above, NanoSIMS is a surface probing technique, with the thickness of the probed surface in the range of a few nanometers. This is important to realize when analyzing thicker samples such as tissues; features seen in a microscope, obtained for example in a transmission or fluorescence mode, may not be “visible” to NanoSIMS if they are buried deeper in the sample. The only way to get to them is by eroding the layers of the sample above them. Although this can be, in principle, done by NanoSIMS itself, clearly this would not be the most efficient way to measure. Therefore, the sample should be prepared preferably in thin sections.
Need for high vacuum
Because the detection of ionized particles eroded from the sample surface is based on their acceleration through a high electric potential, it is essential that the whole process occurs in a high vacuum (<10-7 Pa, <10-9 mbar) to avoid arching. This means that the analyzed samples must withstand such vacuum. Specifically, they should not contain any volatile substances.